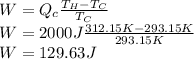Answer:
D. 130 J
Step-by-step explanation:
The coefficient of performance for a machine that is being used to cool, is given by:
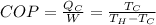
Here
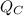 is the heat removed from the cold reservoir, W is the work required, that is, the energy required to remove the heat from the interior of the house,
is the heat removed from the cold reservoir, W is the work required, that is, the energy required to remove the heat from the interior of the house,
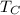 is the cold temperature and
is the cold temperature and
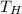 is the hot temperature. Recall use absolutes temperatures(
is the hot temperature. Recall use absolutes temperatures(
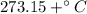 ). Replacing and solving for W:
). Replacing and solving for W: