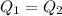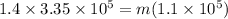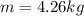Answer:
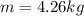
Step-by-step explanation:
As we know that heat required to remove from water to freeze it is given as
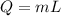
so we have 1.4 kg water is required to freeze
so heat is given as
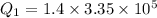
now same heat is removed from ethyl alcohol of mass "m"
the latent heat of fusion for ethyl alcohol is given as
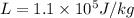
so we have
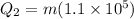
now we know