Answer:
It takes 40 hours to melt the block of ice.
Step-by-step explanation:
According to the principles of radiation and heat transfer respectively:
ΔQ = I(dt)eAcosθ (I = Solar energy density; dt = time taken; e = emissivity; A = Area of block; θ = angle between the sun ray and the horizontal)
ΔQ = mLf (ΔQ = Heat change; m = mass of ice; Lf = Specific latent heat of fusion of ice)
but m = ρV = ρ.A.dx, therefore, the heat transfer equation can be re-written as:
ΔQ = ρ.A.dx.Lf
Lets equate the radiation equation and the modified heat transfer equation, we have:
ρ.A.dx.Lf = I(dt)eAcosθ
ρ.dx.Lf = I(dt)ecosθ (Striking out the area)
Let's make dt the subject of formula,
dt = ρ.dx.Lf /I.e.cosθ
ρ = Density of ice,
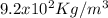
Lf =
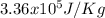
e = 0.050
θ = 32 deg. C
Now, let's substitute the terms:
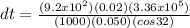
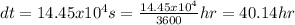
Therefore, the time taken for the ice to completely melt is 40 hours (Two significant figures)