Answer:
a. n = 6,54 moles.
b. CH₃COOH(aq) + NaHCO₃(aq) → CH₃COONa(aq) + H₂O (l) + CO₂(g)
c. 549g of NaHCO₃
d. 7,85L of CH₃COOH
Step-by-step explanation:
a. It is possible to know moles of CO₂ required to inflate the air bag using:
n = PV/RT
Where n are moles; P is pressure (1atm at room conditions); V is volume (160L); R is gas constant (0,082 atmL/molK); and T is temperature (298,15K at room conditions.
Replacing:
n = 6,54 moles.
b. The reaction of CH₃COOH with NaHCO₃ produce:
CH₃COOH(aq) + NaHCO₃(aq) → CH₃COONa(aq) + H₂O (l) + CO₂(g)
c. 1 mol of CO₂ is produced from 1 mol of NaHCO₃, that means 6,54moles of CO₂ are produced from 6,54 moles of NaHCO₃. In grams:
6,54 moles NaHCO₃×
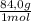 = 549g of NaHCO₃
= 549g of NaHCO₃
d. Again, you require 6,54 moles of CH₃COOH. If your acetic acid solution is 0,833M you need:
6,54moles×
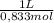 = 7,85L of CH₃COOH
= 7,85L of CH₃COOH
I hope it helps!