Answer:
rom level n = 7 to level n = 3
Step-by-step explanation:
Bohr's model describes the energy levels for the hydrogen atom
En = -13.606 / n²
Where n is an integer with values of 1, 2, 3
An electronic transition occurs between two permitted levels of energy
ΔE =
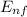 -
-
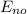
Let's apply these relationships our problem.
Let's start by knowing the energy of level n = 7
E₇ = - 13.606 / 7²
E₇ = - 0.27767 eV
Now let's see what the energy of the emitted photon
E = h f
c = λ f
f = c / λ
E = h c / λ
E = 6.63 10⁻³⁴ 3 10⁸/1005 10⁻⁹
E = 19,791 10⁻²⁰ J
Let's reduce to eV
E = 19,791 10⁻²⁰ (1 eV / 1.6 10⁻¹⁹)
E = 1,237 eV
The possible transitions from this level are towards n = 6, 5, 4,3,2, 1
We must test the different values until we find the right one
Energy of the states
n
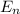
6 -0.378
5 -0.544
4 -0.850
3 -1,512
2 -3,402
1 -13,606
Let's examine the transition n = 7 to n = 6
ΔE = - 0.27767 - (-0.3779)
ΔE = 0.10023 eV
n = 7 to n = 5
ΔE = -0.27767 - (-0.5442)
ΔE = 0.267 eV
n = 7 a n = 4
ΔE = -0.27767- (-0.8504)
ΔE = 0.573 eV
n = 7 a n = 3
ΔE = -0.27767 - (- 1.5118)
ΔE = 1.234 Ev
This is the transition sought, so that the electron goes from level n = 7 to level n = 3