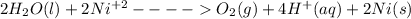Answer:
The following reaction will occur at cathode:
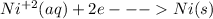
Step-by-step explanation:
The two half reaction during electrolysis of aqueous nickel sulfate will be
a) anode reaction :
Water will undergo oxidation and will evolve oxygen gas at anode as shown in the given reaction:
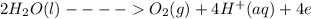
b) Cathode reaction: The reduction of Nickel ion will occur by gain of two electrons as shown in the given equation:
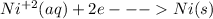
Thus the overall reaction will be: