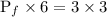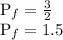Answer:
1.5 atm
Step-by-step explanation:
In this question you have make some assumptions to solve this question:
- The container is air tight, it means no exchange of particle is there.
- The temperature of gas inside container is constant, means it is same in both the conditions.
- The gas is considered as ideal gas.
The equation of ideal gas is:
PV=nRT
P: Pressure of gas
V: Volume of gas or container (as volume of gas is same as volume of container when gas is considered as ideal gas)
n: Number of moles of gas in container
R: Universal gas constant = 8.314 J/mole/K = 0.0821 L·atm/mole/K
T: Temperature
In this question n, R and T as considered as constant, only P and V varies.
The initial conditions are:
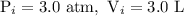
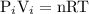
 .........eq(1)
.........eq(1)
The final conditions are:
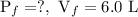
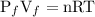
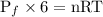 .........eq(2)
.........eq(2)
Since the right side of eq(1) and eq(2) are equal so the left side will also be equal.
Therefore,