Answer:
Concentration of the barium ions =
![[Ba^(2+)] = 0.4654 M](https://img.qammunity.org/2020/formulas/chemistry/college/syjo7f7lc3m7thrry7q5agbhdux5my06dq.png)
Concentration of the chloride ions =
![[Cl^(-)]=0.9308 M](https://img.qammunity.org/2020/formulas/chemistry/college/2uvbiyivi32holckkx1i4kvtma4fzvhedx.png)
Step-by-step explanation:
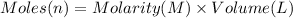
Moles of hydrogen chloride = n
Volume of hydrogen chloride solution = 43.89 mL = 0.04389 L
Molarity of the hydrogen chloride = 0.1355 M
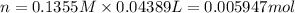
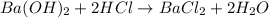
According to reaction, 2 moles of HCl reacts with 1 mole of barium hydroxide.
Then 0.05947 moles of HCl will react with:
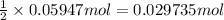 barium hydroxide
barium hydroxide
Moles of barium hydroxide = 0.029735 mol
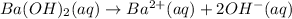
1 mole of barium hydroxide gives 1 mole of barium ion in an aqueous solution. Then 0.029735 moles of barium hydroxide will give:
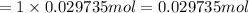
Volume of solution after neutralization reaction :
= 20.0 mL + 43.89 mL = 63.89 mL = 0.06389 L
Concentration of the barium ions =
![[Ba^(2+)]](https://img.qammunity.org/2020/formulas/chemistry/college/nues6tz4ohyof39us2k87lfr5mfa7gnhu5.png)
![[Ba^(2+)]=(0.029735 mol)/(0.06389 L)=0.4654 M](https://img.qammunity.org/2020/formulas/chemistry/college/gch486qj3jfdnpt5q2qsmfnv5fiby75xw3.png)
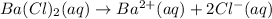
1 mole of barium chloride gives 1 mole of barium ions and 2 moles of chloride ions in an aqueous solution.
Then concentration of chloride ions will be:
![[Cl^-]=2* [Ba^(2+)]=2* 0.4654 M=0.9308 M](https://img.qammunity.org/2020/formulas/chemistry/college/2mtmjdmabjq1i2vb4ja8din3g0fx6wgayv.png)