Answer : The value of
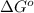 is -166156 J and equilibrium composition should favor products at standard conditions.
is -166156 J and equilibrium composition should favor products at standard conditions.
Explanation :
Now we have to calculate the Gibbs free energy.
As we know that,
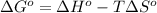
where,
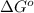 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?
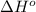 = standard enthalpy = -249 kJ = -249000 J
= standard enthalpy = -249 kJ = -249000 J
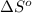 = standard entropy = -278 J/K
= standard entropy = -278 J/K
T = temperature of reaction =
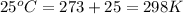
Now put all the given values in the above formula, we get:

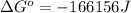
- A reaction to be spontaneous when
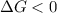 and reaction will be favored in the forward direction that means favored in products.
and reaction will be favored in the forward direction that means favored in products.
- A reaction to be non-spontaneous when
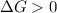 and reaction will be favored in the backward direction that means favored in reactants.
and reaction will be favored in the backward direction that means favored in reactants.
As the value of
 is less than zero that means the reaction is spontaneous and reaction will be favored in the forward direction that means favored in products.
is less than zero that means the reaction is spontaneous and reaction will be favored in the forward direction that means favored in products.
Hence, the value of
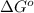 is -166156 J and equilibrium composition should favor products at standard conditions.
is -166156 J and equilibrium composition should favor products at standard conditions.