Answer:
Volume = 746 L
Step-by-step explanation:
Given that:- Mass of copper(II) fluoride = 175 g
Molar mass of copper(II) fluoride = 101.543 g/mol
The formula for the calculation of moles is shown below:

Thus,
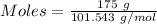
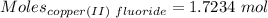
Also,
Considering:

So,,
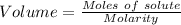
Given, Molarity = 0.00231 M
So,
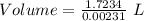
Volume = 746 L