Answer:
Hydroxyl ion concentration is
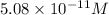 .
.
Step-by-step explanation:
From the given,
pH of the solution = 11.30
pH+pOH = 14
11.30 + pOH = 14
pOH = 14-11.30 = 2.7
The pOH of the solution is 2.7
Concentration of hydroxyl ion:
![2.7= -log[OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/t62zqc5tjgxff5jw8tt42nocswwpmqrc5w.png)
![log[OH^(-)]= -2.7=5.08*10^(-11)M](https://img.qammunity.org/2020/formulas/chemistry/college/n6mfai4v5ys7v3lezr2031sfdifrbudkua.png)
Therefore, hydroxylion concentration is
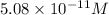 .
.