Answer:
2,196 10⁻⁷ m
Step-by-step explanation:
This is a problem of photoelectric effect, which was explained by Einstein, assuming that the light was formed by quanta of energy that collide with the electors and is described by the expression
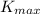 = h f -Ф
= h f -Ф
Where
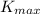 is the maximum kinetic energy of the expelled electrons h is the Planck constant that is worth 6,626 10-34 J s, f is the radiation frequency and Ф is the material's work function.
is the maximum kinetic energy of the expelled electrons h is the Planck constant that is worth 6,626 10-34 J s, f is the radiation frequency and Ф is the material's work function.
For the wavelength of greater wavelength (lower energy) the kinetic energy of the electors must decrease to a minimum of zero.
0 = hf -Ф
hf = Ф
f = Ф / h
f = 9.05 10-19 / 6,626 10-34
f = 1,366 10 15 Hz
Now the speed of the wave is related to the wavelength and frequency
c = λ f
λ = c / f
λ = 3 10⁸ / 1,366 10¹⁵
λ = 2,196 10⁻⁷ m
The result is 2,196 10⁻⁷ m