Answer: The time required to deposit such amount of Mg is 886secs.
Explanation: According to Faraday Law of Electrolysis, the mass of a substance deposited is directly proportional to quantity of electricity passed.
He also stated that;
96500C(1Farday) of electricity is required to deposit 1 mole of any metal.
For
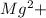 +2e- ==>Mg
+2e- ==>Mg
193000C(2Faraday) of electricity is required to deposit 1mole of Magnesium metal (1 mole of Mg=24g)
Which implies;
19300C will liberate 24g
xCwill liberate 0.110g
Where x is the amount of electricity to deposit 0.110g
x = (19300 × 0.110)/24
x = 884.6C
Recall that Q =It
Where Q is the quantity of electricity, I is the current and t is the time taken
884.6= 0.998 × t
t= 884.6/0.998
t= 886.3secs.
Therefore the time require is 886.3s.