Answer:
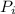 = 2.97 atm
= 2.97 atm
Step-by-step explanation:
The work in thermodynamic processes has several expressions, in the case of isothermal processes the work is
W = n RT ln (
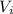 /
/
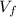 )
)
Where R is the constant of the ideal gases with a value of 8.314 J / mol K and T the absolute temperature
Let's look for the volume ratio
T = 26.4 +273.15 = 299.55k
Ln (
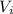 /
/
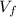 )
)
= W / n RT
Ln(
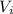 /
/
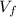 )
)
= 383 / (0.332 8.314 299.55)
Ln(
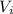 /
/
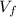 )
)
= 0.4632
(
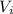 /
/
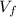 ) = e 0.4632
) = e 0.4632
(
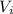 /
/
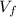 ) = 1,589
) = 1,589
Let's use the ideal gas equation for two volumes
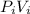 = n R T
= n R T
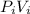 = n RT
= n RT
The temperature is the same because it is an isothermal process, this equation is the same
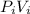 =
=
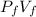
Vi / Vf =
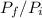
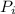 =
=
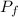
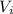 /
/
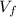
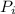 = 1.87 1,589
= 1.87 1,589
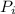 = 2.97 atm
= 2.97 atm