Answer:
only sodium chloride compounds MOST LIKELY products of the reaction between sodium metal and chlorine gas.
Correct answer - C
Step-by-step explanation:
A solid sodium metal reacts with gaseous form of chlorine gas to form a solid sodium chloride.
This formation of sodium chloride arises due to transfer of one valence electron from sodium to chlorine atom and the both atoms get stable octet electronic configuration.
As a result, an ionic compound solid sodium chloride is formed.
The chemical reaction of formation sodium chloride is as follows.
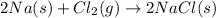
Therefore, only sodium chloride compounds MOST LIKELY products of the reaction between sodium metal and chlorine gas.