Answer:
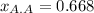
Step-by-step explanation:
Hello!
In this case, since the mole fraction involves the moles of each substance, we first compute them considering the molar masses of acetic acid and acetone to be 60.052 and 58.08 g/mol respectively:
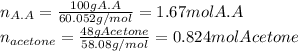
Thus, the mole fraction of acetic acid is computed below:
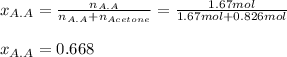
Regards!