Answer:
1) The dilution scheme will result in a 200μM solution.
2) The dilution scheme will not result in a 200μM solution.
3) The dilution scheme will not result in a 200μM solution.
4) The dilution scheme will result in a 200μM solution.
5) The dilution scheme will result in a 200μM solution.
Step-by-step explanation:
Convert the given original molarity to molar as follows.
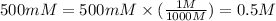
Consider the following serial dilutions.
1)
Dilute 5.00 mL of the stock solution upto 500 mL . Then dilute 10.00 mL of the resulting solution upto 250.0 mL.
Molarity of 500 mL solution:
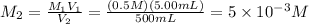
10 mL of this solution is diluted to 250 ml
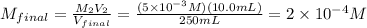
Convert μM :
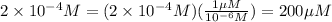
Therefore, The dilution scheme will result in a 200μM solution.
2)
Dilute 5.00 mL of the stock solution upto 100 mL . Then dilute 10.00 mL of the resulting solution upto 1000 mL.
Molarity of 100 mL solution:
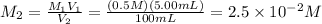
10 mL of this solution is diluted to 1000 ml
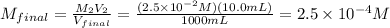
Convert μM :
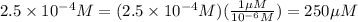
Therefore, The dilution scheme will not result in a 200μM solution.
3)
Dilute 10.00 mL of the stock solution upto 100 mL . Then dilute 5 mL of the resulting solution upto 100 mL.
Molarity of 100 mL solution:
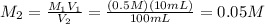
5 mL of this solution is diluted to 1000 ml
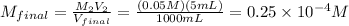
Convert μM :
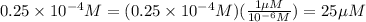
Therefore, The dilution scheme will not result in a 200μM solution.
4)
Dilute 5 mL of the stock solution upto 250 mL . Then dilute 10 mL of the resulting solution upto 500 mL.
Molarity of 250 mL solution:
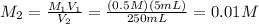
10 mL of this solution is diluted to 500 ml
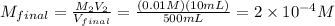
Convert μM :
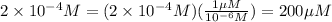
Therefore, The dilution scheme will result in a 200μM solution.
5)
Dilute 10 mL of the stock solution upto 250 mL . Then dilute 10 mL of the resulting solution upto 1000 mL.
Molarity of 250 mL solution:
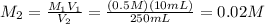
10 mL of this solution is diluted to 1000 ml
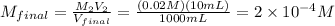
Convert μM :
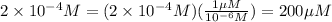
Therefore, The dilution scheme will result in a 200μM solution.