Answer:

Step-by-step explanation:
Given:
- mass of steam,

- temperature of steam,

- temperature of resultant water,

We have,
- latent heat of vapourization of water,
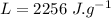
- specific heat capacity of water,

When we cool the steam of 100°C then firstly it loses its latent heat to convert into water of 100°C and the further cools the water.
Now the heat removed from steam to achieve the final state of water:



