Answer:
(a)
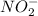 ion has two resonance structures.
ion has two resonance structures.
(b)
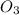 molecule is isoelectronic.
molecule is isoelectronic.
(c) Due to resonance N-O bond is having double bond character.
Step-by-step explanation:
(a)
The Lewis structure of
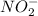 is as follows.
is as follows.
(In attachment)
(b)
Isoelectronic means both species having same number of electrons .
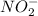 is isoelectronic with
is isoelectronic with
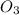 .
.
Both these molecules having 24 electrons and 18 valence electrons.
Hence,
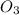 molecule isoelectronic with
molecule isoelectronic with
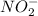 .
.
(c)
The bond length of N-O single bond is more than the N-O double bond.
Due to the resonance structure of
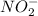 N-O bond has double bond character.
N-O bond has double bond character.