Answer:
The wavelength of the radio wave is 3.003 m.
The energy of the radio wave is
 .
.
Step-by-step explanation:
Frequency of the radio waves, ν = 99.9 MHz =

Wavelength and frequency are related to each other by realtion:
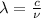
 = Wavelength of the wave
= Wavelength of the wave
c = speed of the light
ν = Frequency of the wave

The wavelength of the radio wave is 3.003 m.
The energy of the electromagnetic wave is given by Planck's equation:
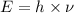
h = Planck's constant =
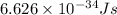
The energy of the radio wave with 99.9 MHz frequency will be:


The energy of the radio wave is
 .
.