Answer: Between 1 and 2.
Step-by-step explanation:
Half life is the amount of time taken by a radioactive material to decay to half of its original value.
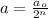 ............(1)
............(1)
where,
a = amount of reactant left after n-half lives = 40
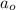 = Initial amount of the reactant = 100
= Initial amount of the reactant = 100
n = number of half lives
Putting in the values we get:
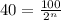
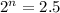
taking log on both sides
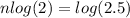
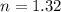
Thus half-lives that have elapsed is between 1 and 2