Answer : The number of moles of ethane required will be 0.166 mole.
Explanation :
First we have to calculate the heat absorbed by water.
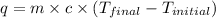
where,
q = heat absorbed = ?
m = mass of water = 851 g
c = specific heat of water =
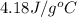
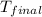 = final temperature =
= final temperature =
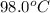
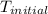 = initial temperature =
= initial temperature =
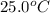
Now put all the given values in the above formula, we get:
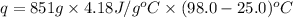
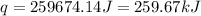 (1 kJ = 1000 J)
(1 kJ = 1000 J)
Now we have to calculate the moles of ethane required.
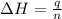
where,
 = enthalpy of combustion of ethane = 1560.7 kJ/mol (standard value)
= enthalpy of combustion of ethane = 1560.7 kJ/mol (standard value)
q = heat absorbed = 259.67 kJ
n = number of moles of ethane = ?
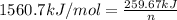
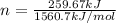
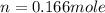
Therefore, the number of moles of ethane required will be 0.166 mole.