Answer:
Equation (I) produced the highest number of carbon atoms.
Step-by-step explanation:
For equation (I):
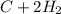 ⇒
⇒
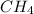 (0.22 moles of
(0.22 moles of
 )
)
2 moles of hydrogen gas will produce 1 mole of methane gas. Therefore, 0.22 moles of hydrogen gas will produced (0.22/2) = 0.11 moles of methane gas.
Similarly, 1 mole of methane gas has one mole of carbon atom and 4 moles of hydrogen atoms. Therefore, 0.11 moles of methane gas will have 0.11 mole of carbon atom.
The number of carbon atom = (moles of carbon atom)*(Avogadro's number)
=
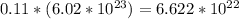 atoms
atoms
For equation (II):
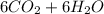 ⇒
⇒
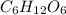
2.25 g of carbon dioxide gas = [2.25g/(44g/mol)] = 0.051 mole of carbon dioxide gas.
In equation (II), 6 moles of carbon dioxide gas produced 1 mole of glucose. Therefore, 0.051 moles of carbon dioxide gas will produce (0.051/6) = 0.0085 moles of glucose.
Similarly, 1 mole of glucose contains 6 moles of carbon atom. Therefore, 0.0085 moles of glucose will contain (0.0085*6) = 0.051 moles of carbon atom.
The number of carbon atom =
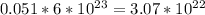 atoms.
atoms.
For equation (III):
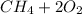 ⇒
⇒
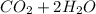
1.25 L of methane gas with density of 0.656 g/L has a mass of (1.25 L*0.656 g/L) = 0.82 g of methane gas.
moles of methane gas = 0.82g/(16g/mol) = 0.051 mol
In equation (III), 1 mole of methane gas produced 1 mole of carbon dioxide gas. Therefore, 0.051 moles of methane gas produced 0.051 moles of carbon dioxide gas.
Similarly, 1 mole of carbon dioxide gas has one mole of carbon atom. Thus, 0.051 moles of carbon dioxide gas will have 0.051 moles of carbon atom.
The number of carbon atoms =
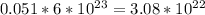 atoms.
atoms.
Therefore, Equation (I) produced the highest number of carbon atoms.