Answer:
The object that is the most dense is 1000 grams per cubic milimeter.
Step-by-step explanation:
A cubic meter is equal to 1,000,000,000 cubic milimeters, 1 kilogram is equal to 1000 kilograms. First, we convert each quantity to grams per cubic milimeter:
a)
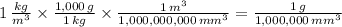
b)
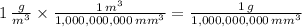
c)
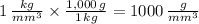
A greater density means that an object is more dense, therefore, the object that is the most dense is 1000 grams per cubic milimeter.