Answer:
The osmotic pressure of blood at 37°C is 7.04 atm.
Step-by-step explanation:
To calculate the concentration of solute, we use the equation for osmotic pressure, which is:
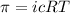
where:
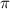 = osmotic pressure of the solution
= osmotic pressure of the solution
i = Van't hoff factor
c = concentration of solute
R = Gas constant =
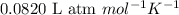
T = temperature of the solution
We have:
Mass of NaCl = 0.90 g
Moles of NaCl =
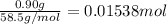
Volume of NaCl solution = V = 100.0 mL = 0.1 L
Concentration of NaCl solution =
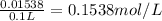
i = 1.8 , T = 37°C = 310.15 K
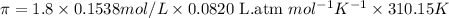
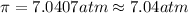
The osmotic pressure of blood at 37°C is 7.04 atm.