The cation is written before the anion in the name and formula of an ionic compound.
Answer: Option B
Step-by-step explanation:
An ionic compounds are a chemical compound at which ions usually held together by an ionic bonds. Typically, a positively charged residue consists of metal cations, while a negatively charged residue consists of an anion or a poly-atomic ion. The ionic compound is mentioned first by a cation after with an anion. The cations have the same name as its element.
For example,
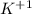 is called the potassium ion but simply 'K' refers potassium atom. Anion is called by the name of the element, removing the end part and adding "ide". For example,
is called the potassium ion but simply 'K' refers potassium atom. Anion is called by the name of the element, removing the end part and adding "ide". For example,
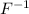 is called fluorine, an element called fluorine. 'ine' was replaced with an 'ide'. To name the compound, the cation and anion names are added together. For example, NaF (sodium fluoride).
is called fluorine, an element called fluorine. 'ine' was replaced with an 'ide'. To name the compound, the cation and anion names are added together. For example, NaF (sodium fluoride).