Answer:
30 moles of CO₂
Step-by-step explanation:
balanced equation ,
CS₂+3O₂ → CO₂+2SO₂
From equation, 3 moles of O₂ are needed to make 1 mol of CO₂.
This means to produce 1 mol of CO₂, we need 3 moles of O₂, then how many moles of CO₂ will be produced from 90 moles of O₂ ?
Use unitary method to solve such problems,
Number of moles of CO₂ that will be produced:
=
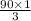
=
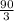
= 30 moles of CO₂