Answer:
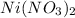
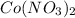
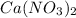
Step-by-step explanation:
The will be a precipitation of the nitrates throughout the reactions.
The nitrates form after the pH was increased. One of the properties to this reaction is the slow solubility of the nitrate salts. The nitrates are strongly binding salts. Hence the slow formation of the salts.