Answer:
63.5 °C
Step-by-step explanation:
The expression for the calculation of work done is shown below as:
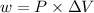
Where, P is the pressure
 is the change in volume
is the change in volume
Also,
Considering the ideal gas equation as:-

where,
P is the pressure
V is the volume
n is the number of moles
T is the temperature
R is Gas constant having value = 8.314 J/ K mol
So,
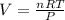
Also, for change in volume at constant pressure, the above equation can be written as;-
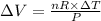
So, putting in the expression of the work done, we get that:-
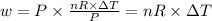
Given, initial temperature = 28.0 °C
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So,
T₁ = (28.0 + 273.15) K = 301.15 K
W=1770 J
n = 6 moles
So,
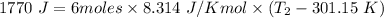
Thus,
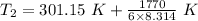
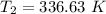
The temperature in Celsius = 336.63-273.15 °C = 63.5 °C
The final temperature is:- 63.5 °C