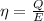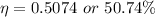Answer:
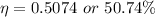
Step-by-step explanation:
Considering the density & specific heat capacity of coffee to be equal to that of water.
GIVEN:
- density
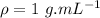
- specific heat
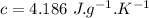
- mass of coffee,
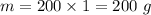
- initial temperature of coffee,
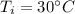
- final temperature of coffee,
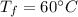
- power rating of oven,
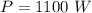
- time taken to reach the final temperature,
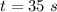
Heat released by the coffee to come to 60°C:
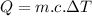
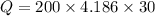
![Q=[tex]\eta=(25116)/(49500)](https://img.qammunity.org/2020/formulas/physics/college/mnf81p1fhwlfnxtk30f75f5h823sgy5yj3.png) \ J[/tex]
\ J[/tex]
Now the energy used by the oven in the given time:
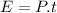
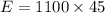
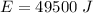
Now the efficiency: