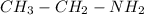Answer:
The condensed structural formula of an ethylamine is
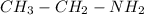 .
.
Step-by-step explanation:
Molecular formula : It is a formula which gives the information regarding different types of atoms and number of atoms in a molecule of an compound.
Condensed structural formula : It is a formula in which the lines are used between the bonded atoms and the atoms are also shown in the structural formula.This formula gives us the picture of positions of the atoms.
Given molecular formula of ethylamine :
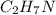
The condensed structural formula of an ethylamine is :