Answer:
Option b: +3 and +6.
Step-by-step explanation:
To calculate the
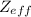 of the valence electron of boron and oxygen, we need to use the next equation:
of the valence electron of boron and oxygen, we need to use the next equation:
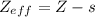
where Z: is the proton number of the atom and s: is the shielding constant
The electronic configuration of the atom of boron and oxygen is the following:
B (Z=5): 1s²2s²2p¹ → number of valence electrons: 3 (2s²2p¹) → number of nonvalence electrons: 2 (1s²)
O (Z= 8): 1s²2s²2p⁴ → number of valence electrons: 6 (2s²2p⁴) → number of nonvalence electrons: 2 (1s²)
Assuming that the shielding constant is approximately equal to the number of the nonvalence electrons, the
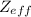 of the valence electron of boron and oxygen is:
of the valence electron of boron and oxygen is:
B:
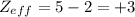
O:
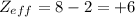
So, the correct option is b, +3 for the boron and +6 for the oxygen.
I hope it helps you!