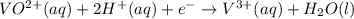Answer:
The balanced half reaction in an acidic medium is :
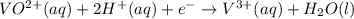
Step-by-step explanation:
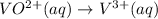
While balancing the half-reaction in an acidic medium, we will first balance oxygen atoms by adding water:
1) Adding 1 water molecule on product side to balance oxygen atoms.
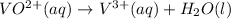
Now balance hydrogen atom by adding
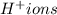 .
.
2) Adding 2 hydrogen ions on reactant side to balance hydrogen atoms.
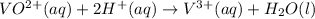
Now balance the charge adding electrons on the side where positive charge is mor.
3) Adding 1 electrons on reactant side to balance charge of the overall reaction:
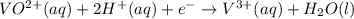
The balanced half reaction in an acidic medium is :