Answer:
Part -A:
Sulfur released is
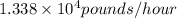 .
.
Part-B:
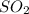 released of per EPA norms is
released of per EPA norms is
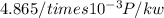 .
.
Step-by-step explanation:
From the given,
The output = 1100 Mw
Efficiency = η = 0.4
Substitute the values in the following
Input = Output / η
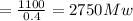
Let's converts between joules to BTU.
1 BTU = 1056 J
Part-A;


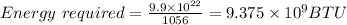
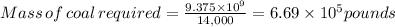
But it has 2% sulfur
The mass of sulfur released
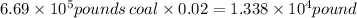
Therefore, released sulfur is
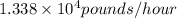 .
.
Part -B;
One pound of sulfur produce two pounds of sulfur dioxide
Initial amount of produced sulfur =
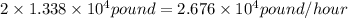
Assuming we added a 80% efficiency then,
Released sulfur dioxide =
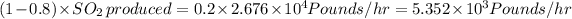
Energy produced in an hour =
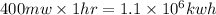
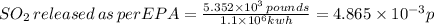
Therefore,
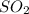 released of per EPA norms is
released of per EPA norms is
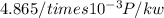 .
.