Answer:
a) 4.01x10⁻³ mol Na₂CO₃
b) 8.02x10⁻³ mol HNO₃
c) 0.241 M
Step-by-step explanation:
2HNO₃ (aq) + Na₂CO₃ (s) ➝ 2NaNO₃ (aq) + H₂O (l) + CO₂ (g)
a) To calculate the moles of Na₂CO₃ we use its molecular weight:
- MW Na₂CO₃ = 23*2 + 12 * 16 * 3 = 106 g/mol
- 0.425 gNa₂CO₃ ÷ 106 g/mol = 4.01x10⁻³ mol Na₂CO₃
b) To calculate the moles of HNO₃ we use the stoichiometric ratio between Na₂CO₃ and HNO₃:
- 4.01x10⁻³ mol Na₂CO₃ *
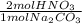 = 8.02x10⁻³ mol HNO₃
= 8.02x10⁻³ mol HNO₃
c) We divide the moles of HNO₃ by the volume in order to calculate molarity:
- 33.25 mL ⇒ 33.25/1000 = 0.03325 L
- 8.02x10⁻³ mol HNO₃ / 0.03325 L = 0.241 M