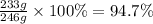Answer:
94.7%
Step-by-step explanation:
The solubility is the maximum amount of solute that can be dissolved in 100 mL (or 100g ) of solvent. Let's consider we have 100 mL of water
At 100 °C, 246 g of solute are dissolved. However, when the solution is cooled to 0 °C, only 13 g of KNO₃ remain dissolved. The mass that crystallizes is:
246 g - 13 g = 233 g
The percentage by mass of the KNO₃ that crystallizes is: