The wavelength of the light is 523 nm
Step-by-step explanation:
Given:
i) First let us determine the frequency:
The energy required to remove an electron = 3.8 x
 J
J
Using Planck's constant the formula for energy, E = hv
3.8 x
 = (6.626 x
= (6.626 x
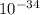 Js) (x)
Js) (x)
Here Planck's constant h = 6.626 x
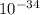
x = 0.5734 x
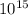
x = 5.734 x
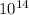
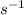
ii) Secondly, let us determine the wavelength:
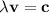
(x) (5.734 x
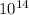
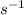 ) = 3 x
) = 3 x
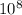 m/s
m/s
Here speed of light c = 3 x
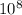 m/s
m/s
x = 0.5231 x
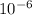
x = 5.231 x
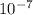 m
m
x = 523 nm