Answer:
433 nm
Step-by-step explanation:
To solve this problem we can use Rydberg's equation:
1 / λ = R *
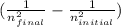
Where λ is the wavelength (in m), R is a constant (1.0974x10⁷ m⁻¹) and n are the initial and final states.
We put the data given by the problem and solve for λ:
1 / λ = 1.0974x10⁷ m⁻¹ *

1 / λ = 2304540 m⁻¹
λ = 4.34x10⁻⁷ m
Converting m to nm:
4.34x10⁻⁷m * 10⁹nm / 1m = 433 nm