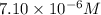Answer:
Concentration of solution B is
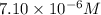
Step-by-step explanation:
Solution A: Total volume of solution A = (9.00+1.00) mL = 10.00 mL
According to law of dilution,
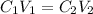
where
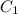 and
and
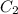 are initial and final concentration respectively.
are initial and final concentration respectively.
 and
and
 are initial and final volume respectively.
are initial and final volume respectively.
Here
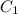 =
=
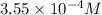 ,
,
 = 1.00 mL,
= 1.00 mL,
 = 10.00 mL
= 10.00 mL
So,
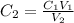 =
=
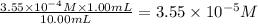
So, concentration of solution A =
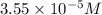
Solution B: Total volume of solution B = (2.00+8.00) mL = 10.00 mL
Similarly as above,
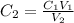 =
=
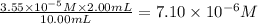
So, concentration of solution B =