The question is incomplete, here is the complete question:
You need to prepare 250. mL of a 0.300 M aqueous solution of sucrose,
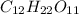 (aq), which is used frequently in biological experiments. Based on your answer above, measure out the amount of solid sucrose in the solution.
(aq), which is used frequently in biological experiments. Based on your answer above, measure out the amount of solid sucrose in the solution.
Answer: The mass of solid sucrose present in the solution is 25.7 grams.
Step-by-step explanation:
To calculate the mass of sucrose, we use the equation used to calculate the molarity of solution:
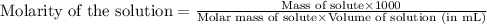
Molarity of solution = 0.300 M
Molar mass of sucrose = 342.3 g/mol
Volume of solution = 250. mL
Putting values in above equation, we get:
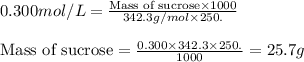
Hence, the mass of solid sucrose present in the solution is 25.7 grams.