Answer:
Mass of P4O6=103.4
P4O10=133.48
Step-by-step explanation:
Balanced reaction is:
8P +8
 ⇒
⇒
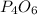 +
+
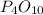
Both reactant completely vanishes as equivalent of bot are equal.
Moles of P=
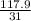 =3.80
=3.80
Moles of
 =
=
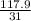 =3.80
=3.80
No. of moles of formed product are equal and is
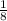 th of mole of any of reactant.
th of mole of any of reactant.
Thus weight of
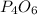 =
=
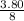 ×220 ≈103.41
×220 ≈103.41
weight of
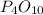 =
=
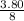 ×284 ≈133.48
×284 ≈133.48