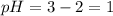Answer:
pH of 0.1M solution : 1
pH of 0.01M solution : 2
The pH changes by 1 if the solution is diluted by a factor of 10
Step-by-step explanation:
pH =
![-log[H+]](https://img.qammunity.org/2020/formulas/chemistry/college/pj3dqrtmay6mw5d2sm82jdrlb9q0znhgyk.png)
where ,
![[H+]](https://img.qammunity.org/2020/formulas/chemistry/college/76w5gmvgo4vgpx256nry8tcjaifgpypl1t.png) is the concentration of
is the concentration of
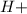 ions
ions
∴
- concentration of
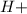 ions is :
ions is :
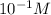
pH of this solution is :
![pH=-log[H+]\\pH=-log[10^(-1)]\\pH=1](https://img.qammunity.org/2020/formulas/chemistry/college/7lu1wmjtv48td73reuou195q1fzmhxr4t0.png)
when the solution gets diluted by a factor of 10 , volume increases 10 times.
As Molarity is inversly proportional to volume , molarity decreases by 10 times.
Thus , molarity becomes : 0.01M
pH of this solution is :
![pH=-log[H+]\\pH=-log[10^(-2)]\\pH=2](https://img.qammunity.org/2020/formulas/chemistry/college/9qplxesjm0t1zyccaydwu9hb1ej3jymbhq.png)
Δ
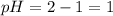
- concentration of
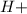 ions is :
ions is :
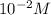
pH of this solution is :
![pH=-log[H+]\\pH=-log[10^(-2)]\\pH=2](https://img.qammunity.org/2020/formulas/chemistry/college/9qplxesjm0t1zyccaydwu9hb1ej3jymbhq.png)
when the solution gets diluted by a factor of 10 , volume increases 10 times.
As Molarity is inversly proportional to volume , molarity decreases by 10 times.
Thus , molarity becomes : 0.01M
pH of this solution is :
![pH=-log[H+]\\pH=-log[10^(-3)]\\pH=3](https://img.qammunity.org/2020/formulas/chemistry/college/mpvacfynec7ut0b7qfxdo47cir63r8i74m.png)
Δ