Answer:
Gas law : Boyle's law
New pressure: 66.24 atm
Step-by-step explanation:
Concept tested: Gas laws (Boyle's law)
We are given,
- Initial pressure, P₁ = 2.86 atm
- Initial volume, V₁ = 8472 mL
- New volume, V₂ IS 365.8 mL
We need to determine the new pressure, P₂
- According to Boyle's law , the volume of a fixed mass of a gas and the pressure are inversely proportional at constant temperature.
- That is,
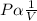
- This means , PV = k (constant)
- Therefore; P₁V₁ = P₂V₂
- Rearranging the formula, we can get the new pressure, P₂
P₂ = P₁V₁ ÷ V₂
= (2.86 atm × 8472 mL) ÷ 365.8 mL
= 66.24 atm
Therefore, the new pressure is 66.24 atm