Answer: Solution A :
![[H_3O^+]=0.300* 10^(-7)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/9yv0chqkzj2qlcg0c82asiapqq9buznoit.png)
Solution B :
![[OH^-]=0.107* 10^(-5)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/nctm8g0v87zprnqyyt5kplmpsvfee57bff.png)
Solution C :
![[OH^-]=0.177* 10^(-10)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/7x79kjtlgbj28wknd1obpw2keubup5h00u.png)
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration and pOH is calculated by taking negative logarithm of hydroxide ion concentration.
![pH=-\log[H_3O^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/f390cegazdnm7uy3e4lyqajx4gquacwg62.png)
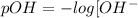
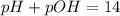
![[H_3O^+][OH^-]=10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/high-school/tzk3kx8tp7r9sh78zjwtrjpmzcwxp4dyv5.png)
a. Solution A:
![[OH^-]=3.33* 10^(-7)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/ow5nguz1ux4a6llwtrghwlxobglgebclw6.png)
![[H_3O^+]=(10^(-14))/(3.33* 10^(-7))=0.300* 10^(-7)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/6xkx8q5vuo3iletjceyipnqyviuaawioxx.png)
b. Solution B :
![[H_3O^+]=9.33* 10^(-9)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/5srnzd73uy42or1vgoktt5ytbbg2e8x38r.png)
![[OH^-]=(10^(-14))/(9.33* 10^(-9))=0.107* 10^(-5)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/82w1ana7wcvujoczzsb30pat9j2fb34te9.png)
c. Solution C :
![[H_3O^+]=5.65* 10^(-4)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/dx0dzglbd5ivszk54a48yisefujufl0m2h.png)
![[OH^-]=(10^(-14))/(5.65* 10^(-4))=0.177* 10^(-10)M](https://img.qammunity.org/2020/formulas/chemistry/high-school/7xbadhptwuev7e1os8f7x78vkn8c2ili5s.png)