Answer:
 = 85.7 ° C
= 85.7 ° C
Step-by-step explanation:
For this exercise we will use the calorimetry heat ratios, let's start with the heat lost by the evaporation of coffee, since it changes from liquid to vapor state
Q₁ = m L
Where m is the evaporated mass (m = 2.00 103-3kg) and L is 2.26 106 J / kg, where we use the latent heat of the water
Q₁ = 2.00 10⁻³ 2.26 10⁶
Q1 = 4.52 10³ J
Now the heat of coffee in the cup, which does not change state is
Q coffee = M
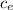 (
(
 -
-
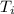 )
)
Since the only form of energy transfer is terminated, the heat transferred is equal to the evaporated heat
Qc = - Q₁
M ce (
 -
-
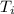 ) = - Q₁
) = - Q₁
The coffee dough left in the cup after evaporation is
M = 250 -2 = 248 g = 0.248 kg
 -Ti = -Q1 / M
-Ti = -Q1 / M
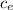
 = Ti - Q1 / M
= Ti - Q1 / M
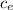
Since coffee is essentially water, let's use the specific heat of water,
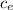 = 4186 J / kg ºC
= 4186 J / kg ºC
Let's calculate
 = 90.0 - 4.52 103 / (0.248 4.186 103)
= 90.0 - 4.52 103 / (0.248 4.186 103)
 = 90- 4.35
= 90- 4.35
 = 85.65 ° C
= 85.65 ° C
 = 85.7 ° C
= 85.7 ° C