Answer:
1.00 M.
Step-by-step explanation:
Hello!
In this case, since the appropriate way to compute the molarity is by dividing the moles of solute by the liters of the solution:
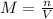
We plug in the given moles and volume to obtain:
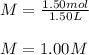
So we prove that the result obtained by him was incorrect.
Best regards!