Answer: During the given fusion process, 4 neutrons are emitted.
Step-by-step explanation:
In a nuclear reaction, the total mass and total atomic number remains the same.
The fusion reaction between californium-249 and nitrogen-15 nuclei, the equation follows:
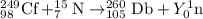
To calculate Y:
Total mass on reactant side = total mass on product side
249 + 15 = 260 + Y(1)
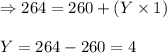
Hence, during the given fusion process, 4 neutrons are emitted.