Answer:
1.05045 kg/m³
Step-by-step explanation:
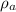 = Density of air = 1.26 kg/m³
= Density of air = 1.26 kg/m³
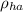 = Density of hot air
= Density of hot air
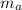 = Mass of balloon = 539 kg
= Mass of balloon = 539 kg
g = Acceleration due to gravity = 9.81 m/s²
v = Volume of air in balloon =
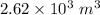
The net force on the balloon will be
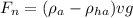
Also
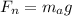
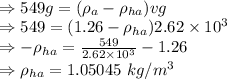
The density of hot air inside the envelope is 1.05045 kg/m³