Answer:
0.35 milli moles of ethanol can be theoretically be produced under these conditions.
Step-by-step explanation:
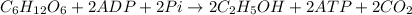
Moles of glucose =
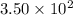 milli mole
milli mole
Moles of ADP = 0.35 milli mole
Moles of Pi = 0.35 milli mole
Moles of ATP = 0.70 milli mole
As we can see that ADP and Pi are in limiting amount which means tat they are limiting reagent. So, the moles of ethanol produced will depend upon the moles of ADP and Pi.
According to reaction, 2 moles of ADP gives 2 moles of glucose.
Then 0.35 milli moles of ADp will give :
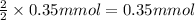 of ethanol
of ethanol
0.35 milli moles of ethanol can be theoretically be produced under these conditions.