Answer:
Response factor A: 8950 L/mg
Response factor B: 4106 L/mg
A = 02497 mg/mL
Step-by-step explanation:
The response factor (F) is defined as the ratio between the signal produced by an analyte and its concentration.
For A:
F(A) = 10919 / 1,22mg/L = 8950 L/mg
F(B) = 5379 / 1,31mg/L = 4106 L/mg
It is possible to obtain relative response factor (RRF) that is the ratio between F(A) and F(B), thus:
RRF = 8950 L/mg / 4106 L/mg = 2,180
RRF could be:
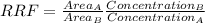
That is:
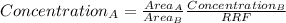
The concentration of B in mg/mL is:
8,18mg / (10,00mL + 50,00mL) = 0,1363 mg/mL
Replacing:
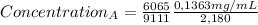
A = 0,04162 mg/mL -In the diluted solution-
The concentration in the unknown solution is:
0,04162 mg/mL ×
 = 02497 mg/mL
= 02497 mg/mL
I hope it helps!